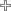Technical data
| Model | Max capacity (l/min) |
Connections BSP inv. |
| T53 | 60 | 3/4" |
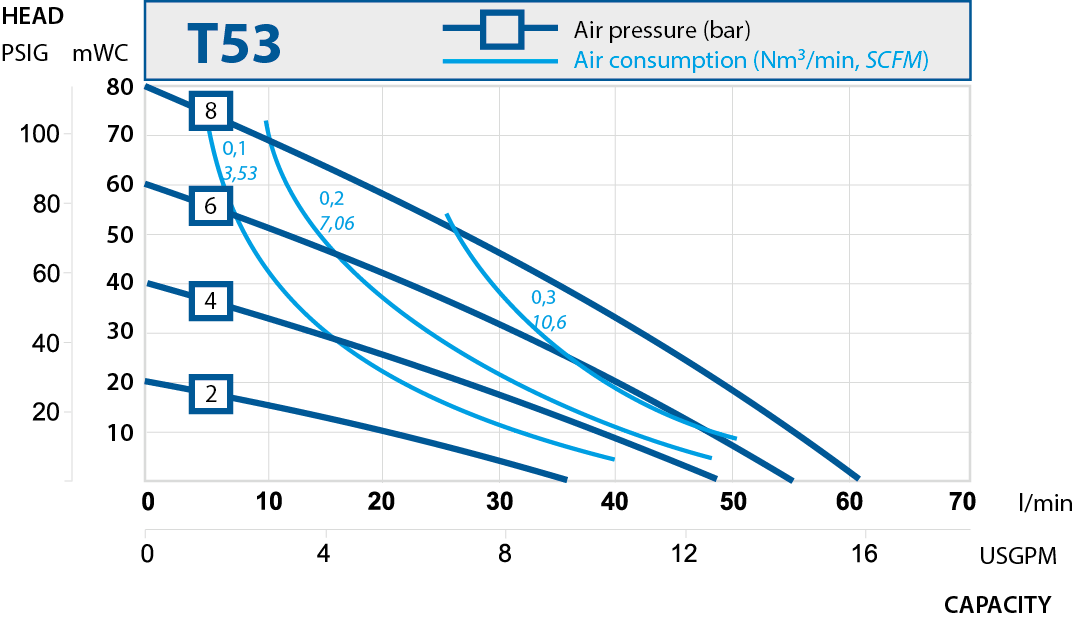
| T103 | 125 | 1" |
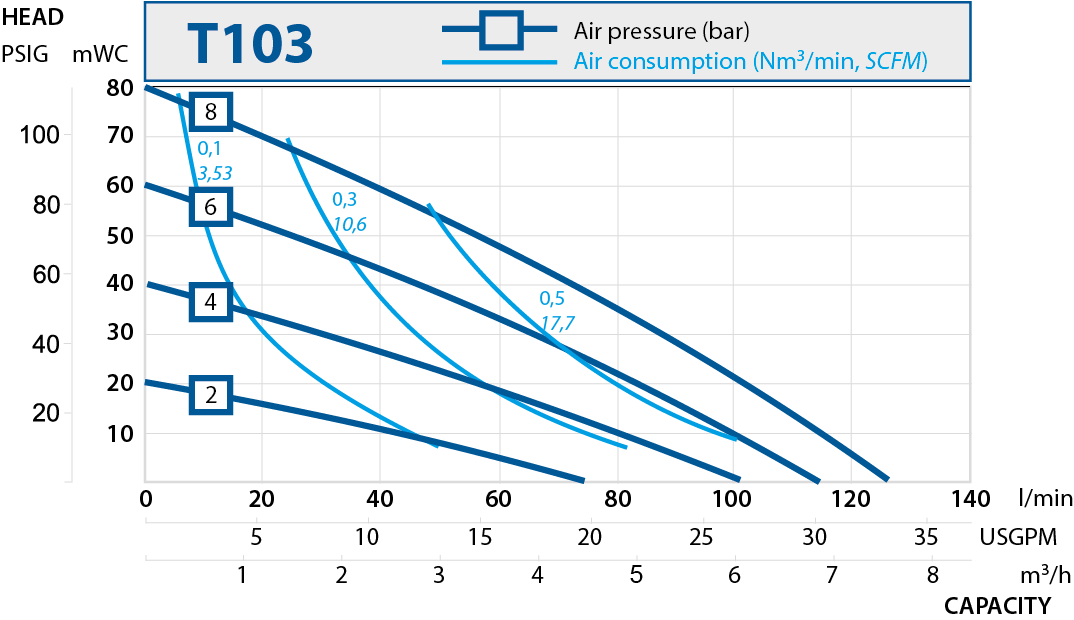
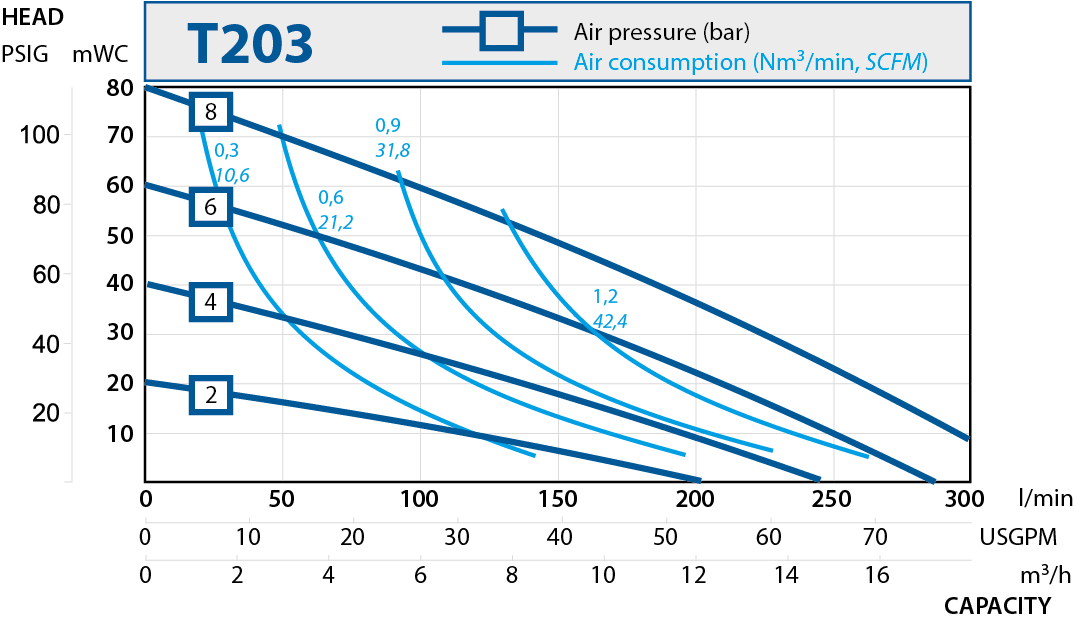
| T203 | 330 | 1 1/2" |
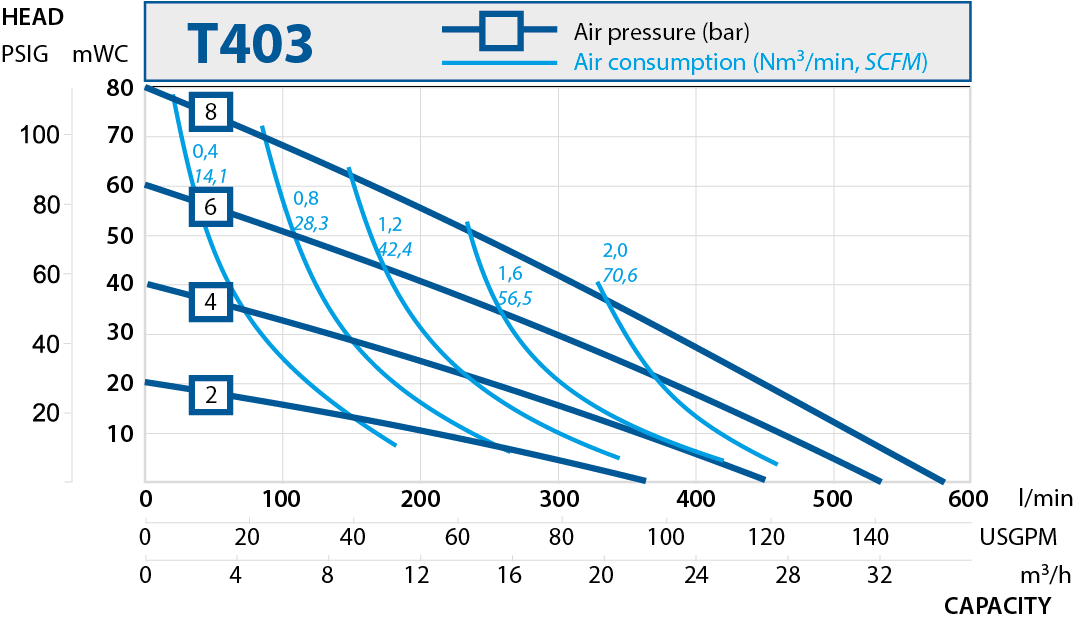
| T403 | 570 | 2" |
This pump series was developed in co-operation with one of the world leading supplier to the biotech market. It serves the biotech- and pharmaceutical industries in numerous applications.
Our unique USP approved (United States Pharmacopoeia) hygienic PTFE or PP pump, features all wetted parts in USP class VI certified materials.
Simplicity
Pump housing with only three parts makes it extremely easy to maintain.
Superior finish
High finish and hygienic approved materials.
Features & Benefits
- Sanitary design
smooth internal surfaces - Inert materials
no contamination of the pumped product - USP class VI
approved materials - Extremely easy to maintain
pump housing with very few components